
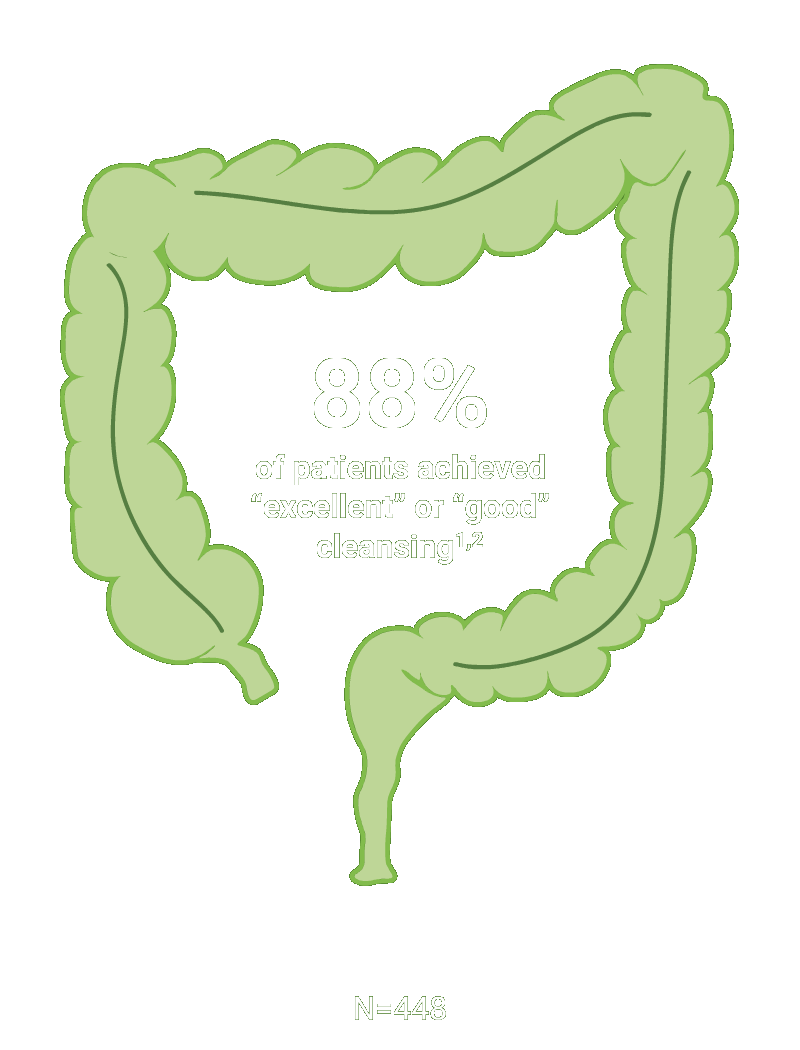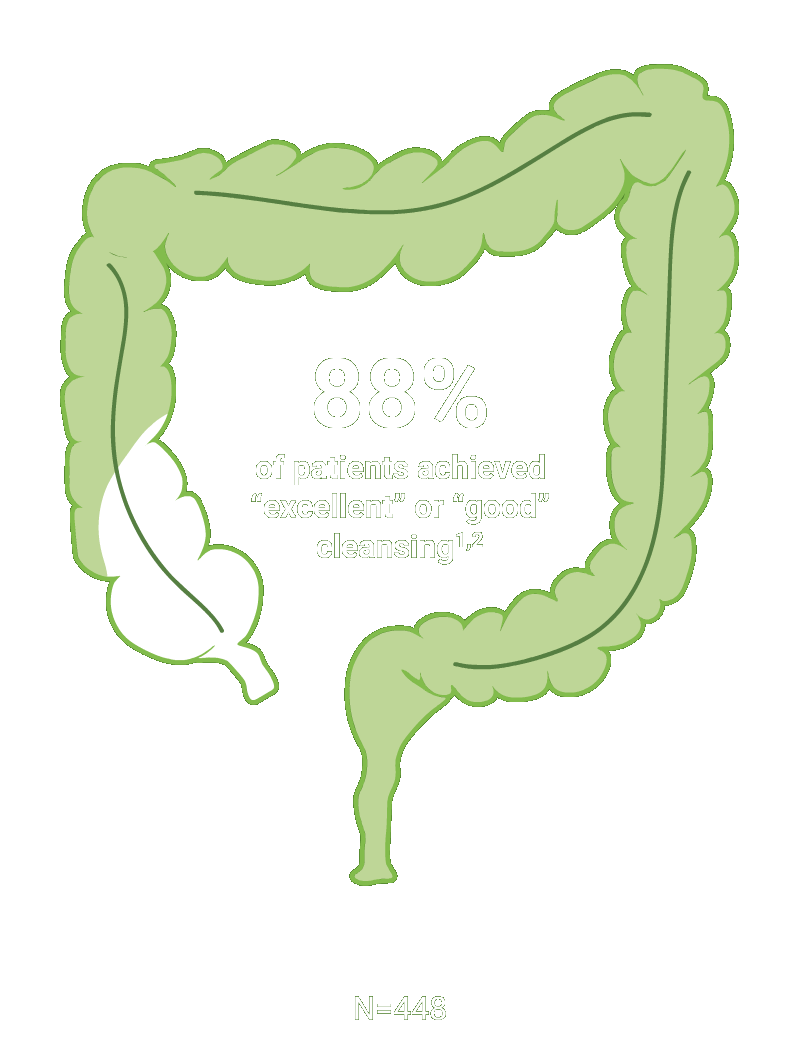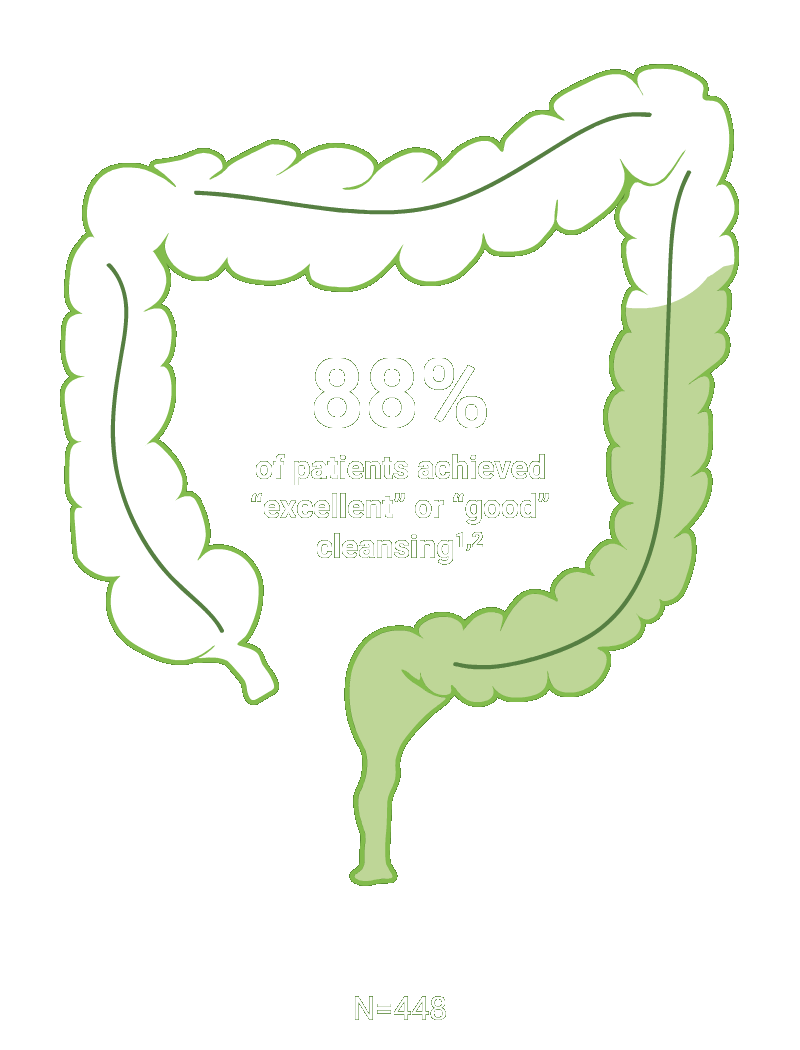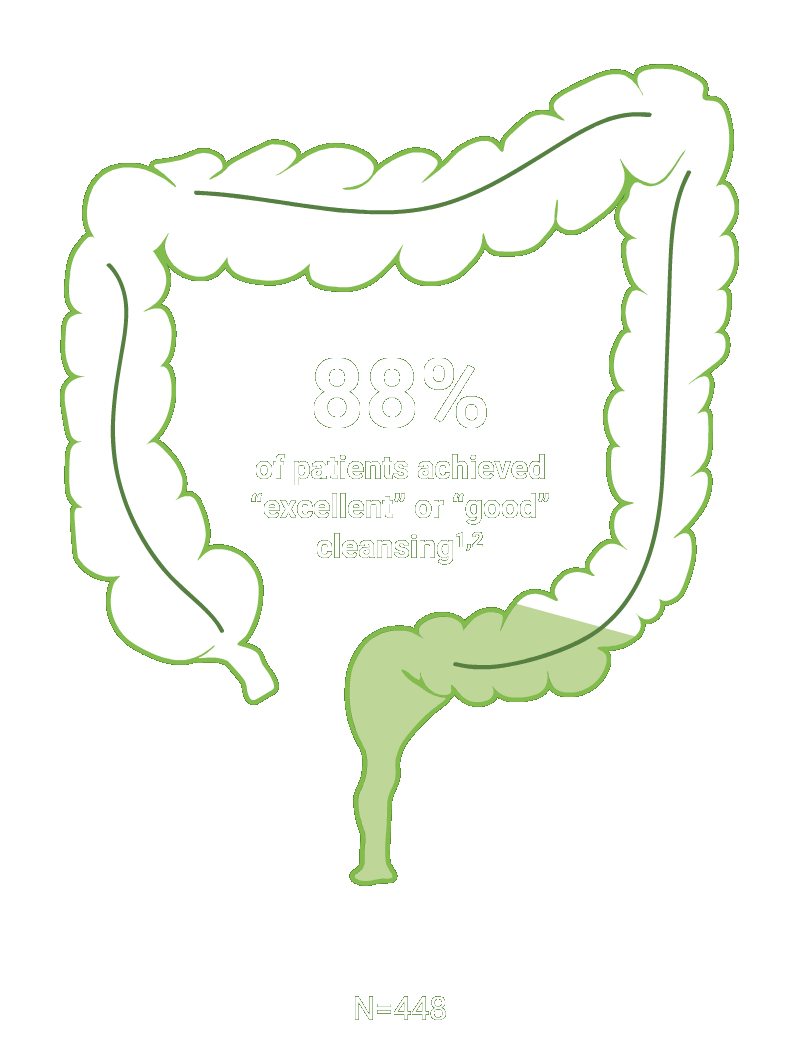
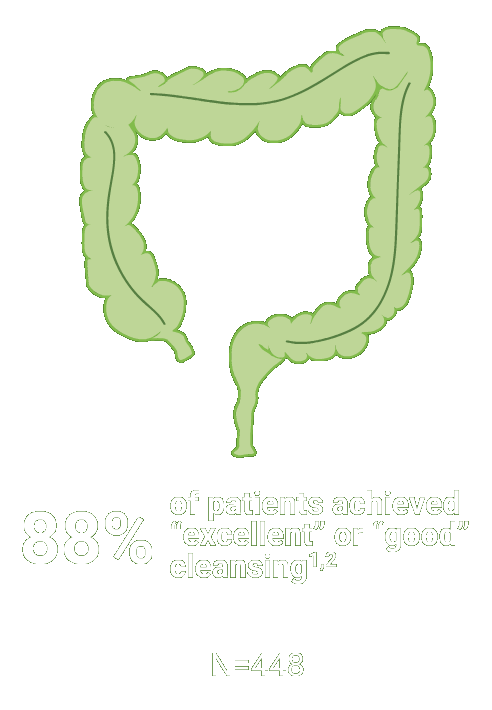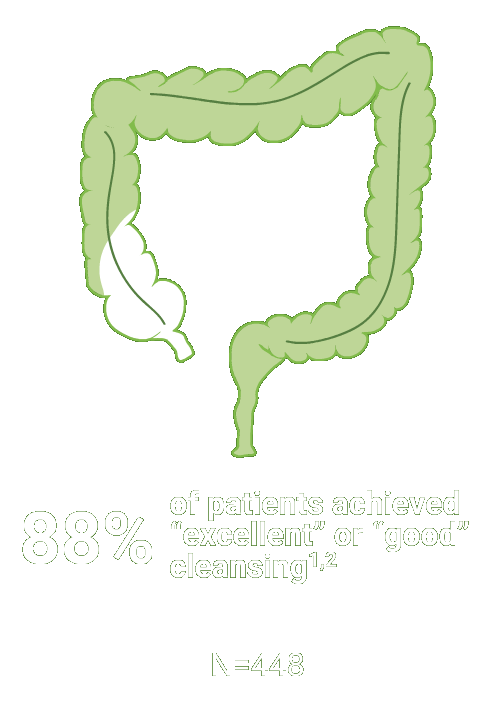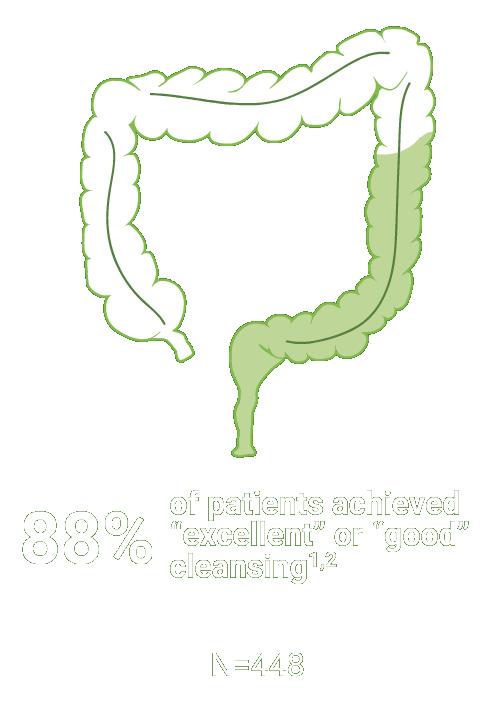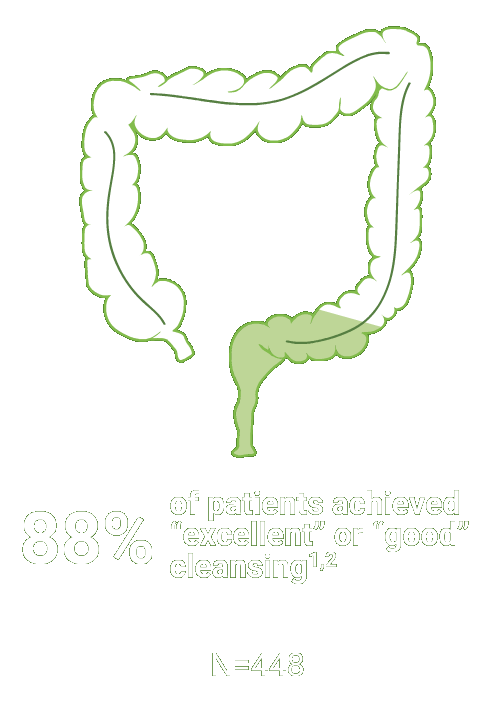
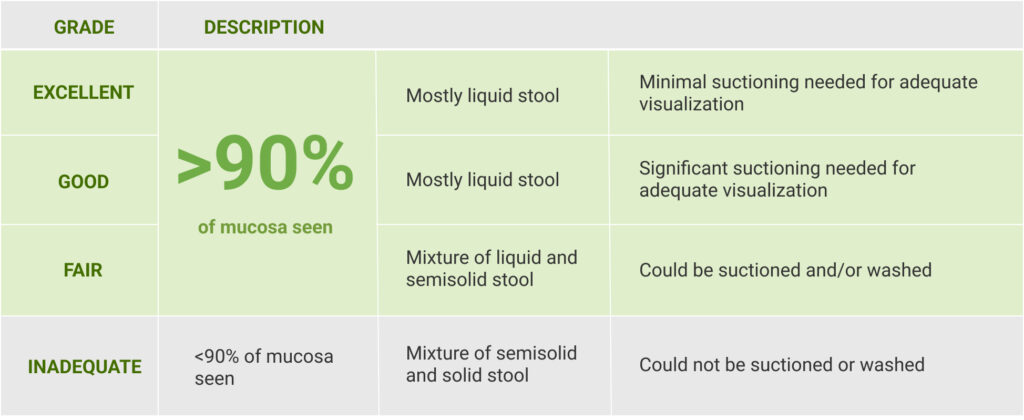
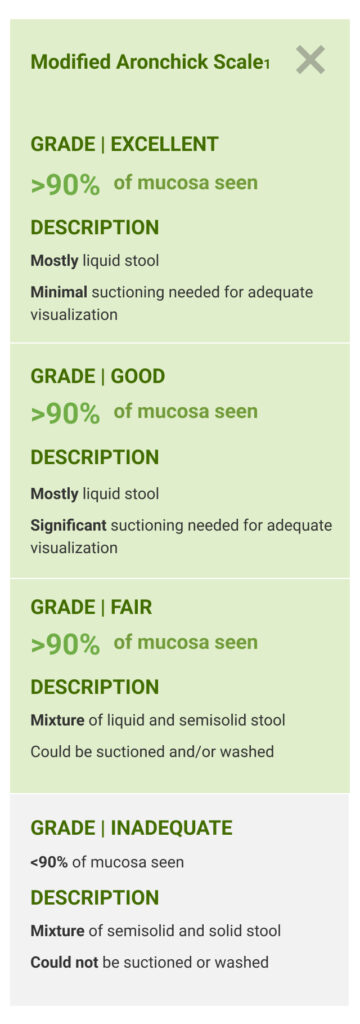
The primary endpoint was the proportion of patients with successful colon cleansing, defined as “excellent” or “good.”
Scoring was performed before washing and suctioning using the Aronchick Scale.

*Trial Design: The safety and efficacy of CLENPIQ were evaluated in a randomized, multicenter, controlled, assessor-blinded trial. CLENPIQ® (sodium picosulfate, magnesium oxide, and anhydrous citric acid) Oral Solution (SPMC) was administered in a split-dose regimen against an oral powder (for reconstitution) (P/MC powder) and efficacy was evaluated using 2 different measurement scales.2 In this trial, CLENPIQ patients were instructed to drink at least five 8-ounce glasses of clear liquid within 5 hours of the first dose of CLENPIQ and at least four 8-ounce glasses of clear liquid within 4 hours of the second dose.2
Modified Aronchick Scale1
See the real-world efficacy of CLENPIQ
Split-dose CLENPIQ demonstrated proven efficacy in cleansing all segments of the colon2†
of patients achieved successful cleansing of the
ascending colon2
SEGMENTAL COLON
Assessed using the Boston Bowel Preparation scale (industry standard)
Secondary efficacy endpoints were the proportion of patients with successful cleansing of the ascending, transverse, and descending colon, defined as a segmental score of “3” or “2.”2
Scoring was performed after washing and suctioning.2


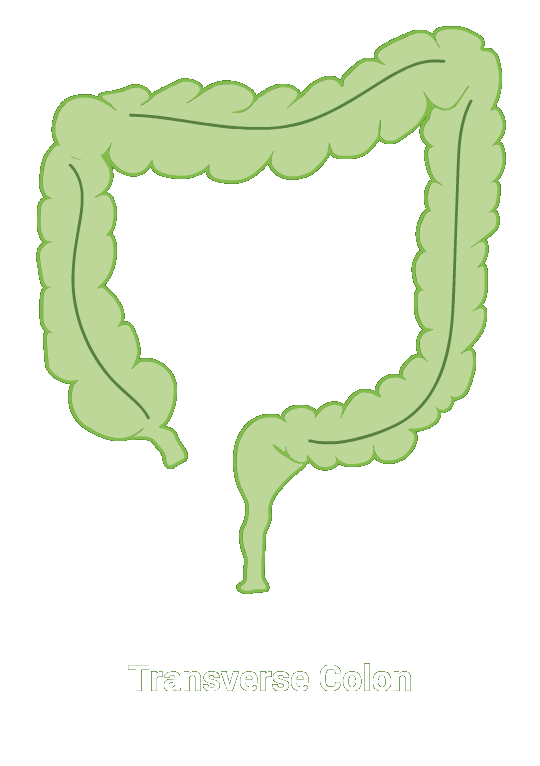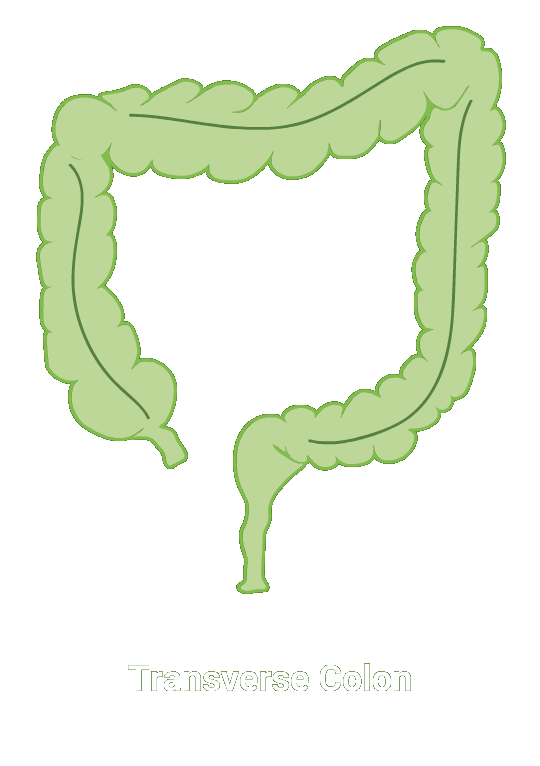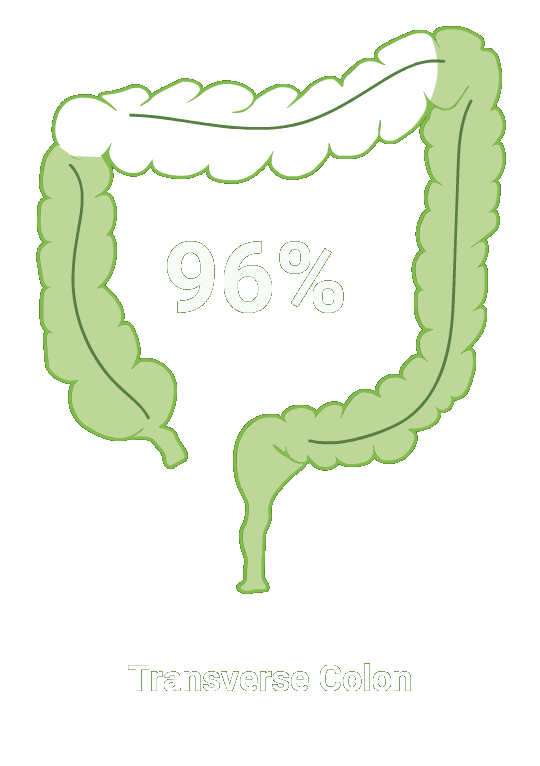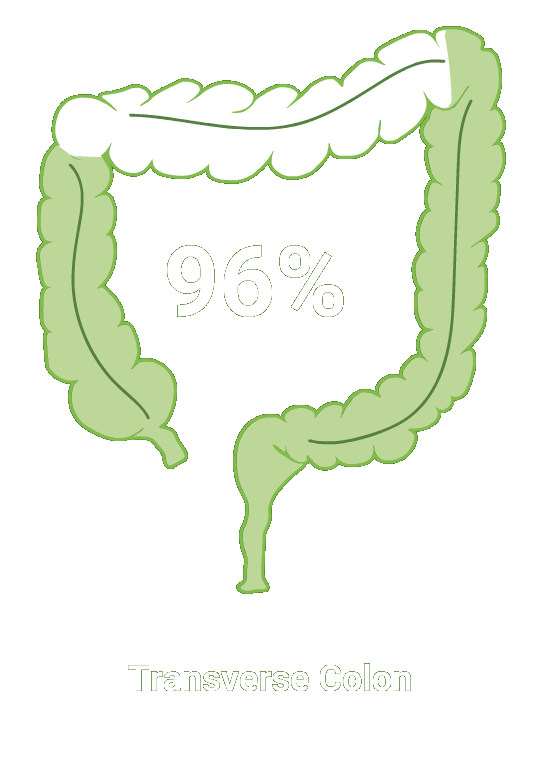
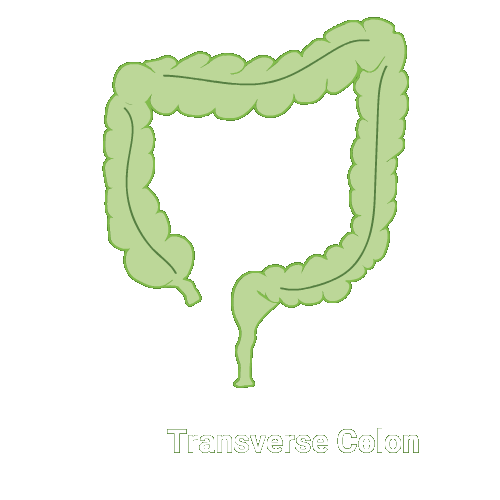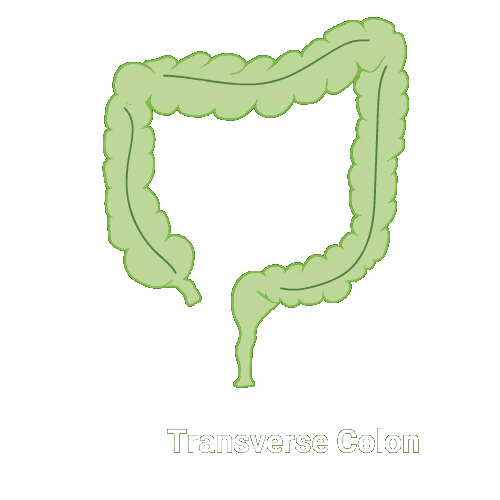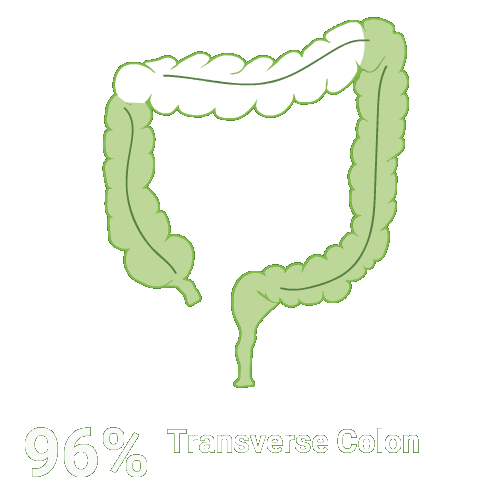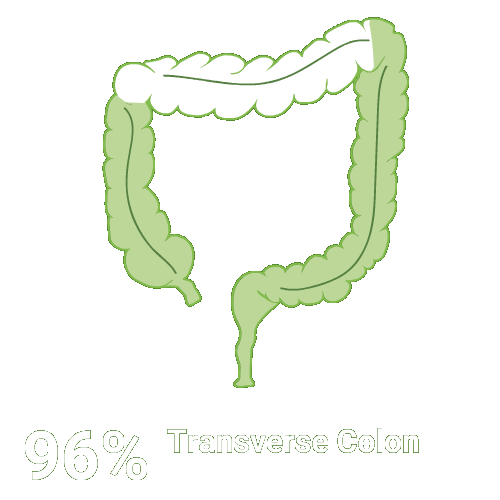


N=448
†Trial Design: The safety and efficacy of CLENPIQ were evaluated in a randomized, multicenter, controlled, assessor-blinded trial. CLENPIQ® (sodium picosulfate, magnesium oxide, and anhydrous citric acid) Oral Solution (SPMC) was administered in a split-dose regimen against an oral powder (for reconstitution) (P/MC powder) and efficacy was evaluated using 2 different measurement scales.2
Split-dose CLENPIQ demonstrated proven efficacy in cleansing all segments of the colon2†
of patients achieved successful cleansing of the
ascending colon2
SEGMENTAL COLON
Assessed using the Boston Bowel Preparation scale (industry standard)




N=448


SEGMENTAL COLON
Assessed using the Boston Bowel Preparation scale (industry standard)
Secondary efficacy endpoints were the proportion of patients with successful cleansing of the ascending, transverse, and descending colon, defined as a segmental score of “3” or “2.”2
Scoring was performed after washing and suctioning.2






†Trial Design: The safety and efficacy of CLENPIQ were evaluated in a randomized, multicenter, controlled, assessor-blinded trial. CLENPIQ® (sodium picosulfate, magnesium oxide, and anhydrous citric acid) Oral Solution (SPMC) was administered in a split-dose regimen against an oral powder (for reconstitution) (P/MC powder) and efficacy was evaluated using 2 different measurement scales.2
Established safety profile1
- The most common adverse reactions (≥2%) observed in the adult CLENPIQ split-dose clinical trial were nausea (3%), headache (3%), hypermagnesemia (2%), abdominal pain (2%), and dehydration or dizziness (2%).1
- Hypermagnesemia levels were transient and not associated with any clinically significant sequelae. Eight of the 9 patients with hypermagnesemia returned to baseline within 24 to 48 hours. One patient returned to baseline by the day 7 follow-up visit.1,2
- 1% of patients taking CLENPIQ experienced vomiting.2

References: 1. CLENPIQ® [Prescribing Information]. Parsippany, NJ: Ferring Pharmaceuticals Inc. 2. Hookey L, et al. Efficacy and safety of a ready-to-drink bowel preparation for colonoscopy: a randomized, controlled, non-inferiority trial. Therap Adv Gastroenterol. 2019;12:1756284819851510. 3. Lichtenstein GR, Cohen LB, Uribarri J. Review article: bowel preparation for colonoscopy the importance of adequate hydration. Aliment Pharmacol Ther. 2007;26(5):633-641. 4. SUPREP® Bowel Prep Kit [Prescribing Information]. Braintree, MA: Braintree Laboratories, Inc. 5. PLENVU® [Prescribing Information]. Bridgewater, NJ: Salix Pharmaceuticals. 6. MoviPrep® [Prescribing Information]. Bridgewater, NJ: Salix Pharmaceuticals.
